Thermal Energy And Temperature Relationship
Thermal energy always moves from an object with a higher temperature to an object with a lower temperature.
Thermal energy and temperature relationship. The energy in the thermal store of an object is related to the state of motion of its particles and the number of particles a higher thermal energy follows from a larger number of faster moving particles. Temperature is a measurement of the average kinetic energy of the molecules in an object or a system. If you re seeing this message it means we re having trouble loading external resources on our website. Heat is the transfer of thermal energy between objects that have different temperatures.
When scientists need to think about abstract ideas they often use a model. Temperature and thermal energy. First however you will need to know just a little bit about temperature and thermal energy. As the air becomes warmer heat is transferred between molecules and kinetic energy is created which produces thermal energy.
Specific heat is the amount of energy in joules. Thermal energy that flows from something at a higher temperature to something at a lower temperature and it only travels in one. Thermal energy is an extensive quantity since it depends upon the number of particles or mass of substance present. We can say that 100 g of hot water contains more energy not heat than 100 g of cold water.
What are temperature and thermal energy. As the molecules move faster to transfer heat the temperature also increases. What is the relationship between thermal energy and temperature. The more mass an object has the more thermal energy because it has more particles.
Thermal energy and temperature. These latter two forms of thermal energy are not really chaotic and do not contribute to the temperature. This difference reflects the important distinction between energy and temperature. An example of the way temperature and thermal energy work is how the sun heats air located above the surface of the earth.
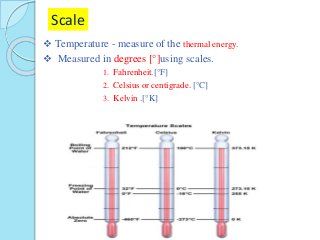
Energy is measured in joules and temperature in degrees. What heat means in thermodynamics and how we can calculate heat using the heat capacity. Kinetic energy is the energy that an object has because of its motion. The concepts of thermal energy and temperature play a very important role in fields such as heat and thermodynamics mechanical engineering physical chemistry physics astronomy and various other fields.